Total alkalinity in pool or spa water provides buffering so that pH does not swing in and out of the proper range in response to sanitizer addition, bather load or other factors. With total alkalinity levels too low, there will not be enough buffering, and the pH may quickly drift out of the proper range.
Maintaining total alkalinity depends on keeping the concentration of CaCO3 (calcium carbonate) within the recommended range, thus reducing the tendency of pool and spa water to scale or etch surfaces. The ANSI/APSP/ICC11 2019 American National Standard for Water Quality in Public Pools and Spas by the Pool & Hot Tub Alliance states that total alkalinity shall be maintained between a minimum of 60 ppm and a maximum of 180 ppm as CaCO3.
RELATED: Total Dissolved Solids: The Facts
Ideally, total alkalinity should be maintained between 80 and 100 ppm as CaCO3 where electrolytic chlorine generators, calcium hypochlorite, lithium hypochlorite and sodium hypochlorite are used, because these sanitizers cause the pH to rise. Where sodium dichlor, trichlor, chlorine gas and bromine are used, the ideal range is between 100 and 120 ppm as CaCO3, because these sanitizers will cause the pH to drift downwards.
Polyhexamethylene biguanide (PHMB) sanitizer efficacy in controlling bacteria is unaffected by total alkalinity changes, and the sanitizer does not impact total alkalinity, but it is still important to maintain total alkalinity in biguanide treated pools to maintain water balance and clarity in pools. The total alkalinity in biguanide pools has a recommended range of 80 to 150 ppm.
MEASURING AND ADJUSTMENT
Total alkalinity is most often measured using test kits or test strips. Carbonate alkalinity is calculated from total alkalinity, cyanuric acid (CYA) and pH (see calculation below). Carbonate alkalinity should always be used when calculating LSI. Total alkalinity of pool or spa water should be corrected before adjusting pH or sanitizer levels.
RELATED: All About Alkalinity
To reduce total alkalinity, acid is added to the water. Approximately 2.1 lbs of sodium bisulfate (94%) or 1.6 pints of muriatic acid (31%) will reduce the total alkalinity of 10,000 gallons of water by 10 ppm.
Sodium bicarbonate is used to increase total alkalinity. Approximately 1.5 lbs of sodium bicarbonate (100%) will raise the total alkalinity of 10,000 gallons of water by 10 ppm.
Calculating Carbonate Alkalinity
Dichlor and trichlor sanitizers release CYA, which serves to stabilize the chlorine sanitizer. CYA stabilizer may be added separately as well. The cyanurate system is a weak buffer and will contribute to the total alkalinity concentration.
The approximate carbonate alkalinity is often calculated by subtracting onethird (1/3) of the CYA concentration in ppm from the total alkalinity concentration in ppm as CaCO3. A more accurate calculation of carbonate alkalinity is given below:
1. Measure the pH.
2. Measure total alkalinity (Measured TA).
3. Measure CYA concentration. If the CYA is 90 ppm or greater, it may be necessary to dilute the pool water sample with tap water to get an accurate reading.
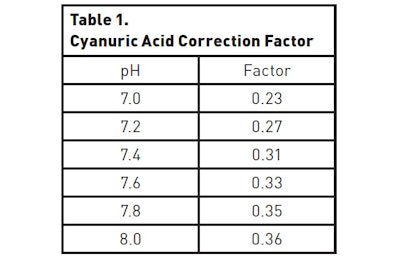
4. Multiply the Cyanuric Acid Correction Factor in Table 1, based on the pH of the water, by CYA concentration to adjust the CYA.
5. Subtract the adjusted CYA from the total alkalinity to get the carbonate alkalinity.
6. Formula: Measured TA – (CYA x Cyanuric Acid Correction Factor) = Carbonate Alkalinity
Example:
pH is 7.4. Total Alkalinity measurement
(Measured TA) is 110 ppm.
Cyanuric Acid level is 100 ppm.
Cyanuric Acid Correction Factor at pH 7.4 is
0.31. (From Table 1)
Using the formula:
110 ppm – (100 ppm x 0.31) =
110 ppm – 31 ppm =
79 ppm Carbonate Alkalinity
References:
1. ANSI/APSP/ICC-11 2019 American National Standard for Water Quality in Public Pools and Spas, Pool and Hot Tub Alliance, 2019
2. "APSP Service Tech Manual, Basic Pool & Spa Technology," The Association of Pool and Spa Professionals, Alexandria, Virginia, Third Edition
3. Wojtowicz, J.A. "Treatment of Swimming Pools, Spas, and Hot Tubs," Kirk – Othmer Encyclopedia of Chemical Technology, Fourth Edition, Vol. 25, John Wiley and Sons, Inc., New York, NY, pp 569-594, 1998
4. Wojtowicz, J.A. "The Carbonate System in Swimming Pool Water," Journal of the Swimming Pool and Spa Industry, 3(1) (2001):54-59, 20015. RWQC "Water Balance Indexes" Fact Sheet, Pool & Hot Tub Alliance, 2017, https://www. phta.org/pub/?id=0944F15C-1866-DAAC99FB-94B844527165
This article first appeared in the July 2021 issue of AQUA Magazine — the top resource for retailers, builders and service pros in the pool and spa industry. Subscriptions to the print magazine are free to all industry professionals. Click here to subscribe.











































