You would think that adding muriatic acid, dry acid, soda ash or even bicarb for adjusting pH and alkalinity would be a simple calculation. This is reinforced by the fact that there are charts and tables listing the amount of acid, soda ash or bicarb needed to lower or raise pH and alkalinity. This leads you to believe that the amount of acid needed to change pH from 8.0 to 7.5 will be the same regardless of the alkalinity, CYA, borate, calcium hardness, salt, and water temperature. However, all of these water conditions have an effect on the amount of acid needed.
Hydrogen Ions Are Key
With the help of Richard Falk of WaterGuru, aka chem geek of Trouble Free Pool, we have created a very accurate spreadsheet that takes into consideration all the sources of hydrogen ion (H+) change in pool and spa water. There are equilibrium shifts for CYA, borate, carbonate, ionic strength, calcium hardness and temperature plus the starting pH and alkalinity. (I hope to create an app that you can use on your cell phone but it may take some time for programming and to develop a user-friendly interface.)
You probably already know that the percentage of HOCl (hypochlorous acid — the killing form of chlorine in water) and OCl_ (hypochlorite ion — the non-killing form of chlorine in water) are determined by the pH. At pH 7.5 there is about 50% HOCl and 50% OCl_. Raise the pH to 8.0 and the percentages are 22% HOCl and 78% OCl_. When HOCl converts to OCl_ a hydrogen ion (H+) is released according to this reaction:
HOCI ↔ OCI– + H+
Hypochlorous acid is in equilibrium with hypochlorite ion and hydrogen ion
The H+ is either liberated or used depending on the pH of the water. When it is liberated, it is then counted as part of the total hydrogen ions in the water which we call pH. The more H+, the lower the pH and the less H+, the higher the pH. This is just one example of a hydrogen ion source in the water.

Another example is carbonate to bicarbonate:
HCO3– ↔ CO32– + H+
Bicarbonate ion is in equilibrium with carbonate ion and hydrogen ion
Calculating the amount of hydrogen ion released or used is quite complex and there are about 30 different equilibrium reactions to consider with each requiring a separate calculation.
Acid Amount for Lowering pH Varies with CYA, Borate and Temp
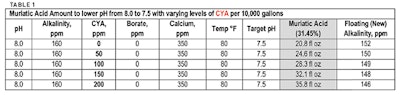
Let's start with a simple lowering of pH with muriatic acid and see how some water conditions change the amounts of acid needed. I have used a TDS of 1,000 ppm for each calculation.

It should be obvious that the amount of acid needed to make a change of pH from 8.0 to 7.5 is very different depending on only CYA, borate and temperature. The amount of muriatic acid is from 18.8 fl oz with 0 ppm CYA, 0 ppm borate, and temperature of 100° F to 61.1 fl oz with 150 ppm CYA, 50 ppm borate and temperature of 100° F. In other words, it takes 3.25 times more acid to lower pH when CYA, borate and temperature are high. And, the amount of acid used will have varying effects on alkalinity.
You can also see that adjusting the water to a pH of 7.5 (if alkalinity is high also), will only lower alkalinity by 7 ppm to 14 ppm. So, you end up with an alkalinity that is still too high. From a water balance standpoint and from a practical standpoint, a high alkalinity will continuously raise the pH. You will always be adding acid to a pool that has high alkalinity.

It should also be apparent that using a chart, table or even a water balance app that gives acid amounts to lower pH and alkalinity is questionable unless the table or chart includes adjustments for CYA. Borate, temperature, salt and calcium levels in the water.
Let's take a look at the amounts of acid to lower total alkalinity to a target of 100 ppm and see how much acid it takes and what it does to the pH.
I would bet that you were not expecting the amount of acid to remain constant. We need the same amount of acid to lower alkalinity because our starting alkalinity of 140 ppm and our ending alkalinity of 100 ppm is the same. Acid lowers alkalinity be the same ppm regardless of starting pH. If the starting pH is changed from 7.5 to 8.0, it would still take 102.6 fl oz to change alkalinity from 140 ppm to 100 ppm. The difference will be that the new pH after adding the acid will be 6.97 because we started at 8.0. The new pH changes because of the effect of CYA. So, the pH is a little higher with increasing levels of CYA. CYA is a pH buffer and borate is a pH buffer.

It takes 2.56 fl oz of muriatic acid to lower total alkalinity by 1.0 ppm in 10,000 gallons. From this information, you can calculate the amount of acid necessary to lower total alkalinity in any pool.
2.56 fl oz × (Pool Gallons/10,000) × Alkalinity Decrease = Amount of Muriatic Acid in fl oz
So, if you have an 18,000-gallon pool with an alkalinity of 140 ppm and you want to lower alkalinity to 100 ppm, here is the math:
2.56 fl oz × (18,000/10,000) ×
(140 –100)
2.56 × (1.8) × 40 = 184.3 fl oz
And there are 128 fl. oz. in one gallon, so:
184.3/128 = 1.44 gallons
What you can see is that we can lower alkalinity to the target of 100 ppm and then aerate and cause turbulence and we can raise only the pH to 7.5 with no change to alkalinity. Perfect water quickly.

Who Knows How Much Soda Ash is Needed to Raise pH?
Determining the amount of soda ash needed for raising pH in pool water is difficult to calculate for the same reasons as finding the acid amount. There are 30 or so variables that affect the calculation. In addition, there are only a few charts or tables readily available showing how much soda ash is needed to raise pH. And I am certain that the charts do not give any information about the resulting or new alkalinity after addition. And they certainly would not take into consideration the 30 variables that we have mentioned.
There are two important facts that you should remember when deciding to raise pH with soda ash or bicarb. First, sodium bicarbonate should not be used to make a pH change. Raising total alkalinity by 20 ppm with bicarb in 10,000 gallons of water would take 44.8 oz of bicarb and it would raise pH by only 0.072 from 7.5 to 7.572. Another way to look at this is that raising alkalinity by 100 ppm in 10,000 gallons of water would require the addition of 228.1 oz or 14.26 lbs of bicarb and it would only raise pH by 0.225 from 7.5 to 7.725. By contrast, if you added 14 lbs. of soda ash it would raise the pH to 9.6 and increase alkalinity by 162 ppm.
Second, you can raise only the pH in the water by aeration and turbulence. This is important because all you need to do is aim the returns up so they break the surface of the water, turn on spillovers, fountains or other features that create aeration and turbulence. A negative edge pool and spa with jets will continuously raise the pH. You can even use a submersible drain pump, set it on the top step and shoot the return water up into the air and let it fall into the pool. This will raise the pH fast.
Soda ash raises pH and alkalinity. Usually, if you try to use it to raise pH, you will raise the alkalinity too high and have to add acid to adjust it back down.

Table 8 will give you some idea about using soda ash for pH adjustment.
As you can see, it can take 2.4 times more soda ash to change pH from 6.8 to 7.5 when the CYA goes from 0 ppm to 200 ppm (36.4 oz versus 88.6 oz). And when CYA goes from 0 to 200 ppm and with borate at 50 ppm, (36.4 oz versus 100.0 oz) it would take 2.75 times more acid. In addition, the alkalinity in most cases would now be too high.
One option is to raise pH some with soda ash and then adjust alkalinity with bicarb. However, you are again faced with not knowing how much soda ash to add to raise the pH. The spreadsheet will calculate the amounts of bicarb and soda ash and muriatic acid to do this.
The other option (which is far better) is to adjust the alkalinity to the target of 90-100 ppm with bicarb (or acid depending on starting pH and alkalinity) and then raise pH by aeration and turbulence. The amount of bicarb needed to raise alkalinity is easy to calculate. It takes 2.24 oz of bicarb to raise alkalinity by 1.0 ppm in 10,000 gallons of water. Therefore:
2.24 oz × (Pool Gallons/10,000) × Alkalinity Increase = Bicarb in ounces
So, if you have an 18,000-gallon pool and you want to raise alkalinity from 60 to 100 ppm, here is the math:
2.24 oz × (18,000/10,000) × (100 – 60) = oz of soda ash
2.24 oz × 1.8 × 40 = 161.28 oz of soda ash
and because there are 16 oz in a pound:
161.28/16 = 10.08 lbs of soda ash

Then you can aerate the water and raise the pH to 7.5 with no change to alkalinity. Perfect water at a pH of 7.5 and a TA of 100 ppm. And if you use a submersible pump and shoot the discharge high into the air you can raise pH in about 30 minutes from 7.0 to 7.5 depending on pool gallons and flow rate of the pump.
How Do You Know if the Water Balance App or Calculator is Right?
Water balance calculators whether on-line or an app have limited ability, and most are created by using the acid and alkalinity tables that have been around for years that are seriously wrong or inadequate. Here is an easy test to figure out if the recommendations you are getting from your water balance app is correct or helpful.
Enter gallons of 10,000, a pH of 8.0. TA of 140 ppm, CYA of 100 ppm, borate 0, a temp of 80°, a calcium of 350 ppm and TDS of 800 ppm. (Just enter the conditions that your app asks for.) You should also input a goal or target pH of 7.5 and 100 ppm alkalinity.
Your program will hopefully recommend an amount of acid that is near 100 fl oz. which will lower the alkalinity to 100 ppm. If not, then it should recommend an amount of acid near 31 fl oz which should lower pH to 7.5.
If your program has an acid dose of near 100 fl oz, then did it give you the new pH? If not, your pH will now be between 6.6 and 6.8 as you can see from table 6. So if you now input the new pH of 6.6 and an alkalinity of 100 ppm into your program, what is the recommendation? It will probably tell you to add soda ash to raise pH. The amount will be about 100 oz. This will make the pH 7.5 but your new alkalinity will be 170 ppm! Is this the right advice? Did your program balance your water?
If your program had an acid dose of near 30 fl oz it means that it is lowering the pH to 7.5 from 8.0. The pH is now okay but what will the alkalinity be? Did your program tell you the new alkalinity? If not, the new alkalinity will be 130 ppm.
Now if you enter a pH of 7.5 and an alkalinity of 130 ppm, what does it say to do? It will tell you to add more acid, but this will lower your pH farther. If you add a dose of acid to get to 100 ppm alkalinity, your pH will be near 6.8. Now if you input pH 6.8 and TA of 100 ppm, what does the program say to do? Add soda ash. Same problem. If you add the right amount of soda ash to raise pH to 7.5 you will have an alkalinity of about 160 ppm. This is higher than you started with.
This is the problem with most online apps, tables and phone apps. They really do not balance the water to a pH of 7.5 and an alkalinity of 90-100 ppm and none of them recommend aeration and turbulence to raise the pH.
Soon there will be an app that you can use to balance your pH and alkalinity. In the meantime, I hope the above charts and other information will be of help.
Since joining the industry back in 1973, Robert Lowry has started two chemical companies (Robarb and Leisure Time Chemicals), authored 12 books, co-founded Service Industry News and developed 111 products. He has worked as an independent consultant since 1995. His seminars on water chemistry in pools and spas are widely considered the gold standard for water treatment education within the industry. Lowry's re-examination of the CYA/chlorine relationship began over a decade ago, and has been a key component of the debate on CYA and its proper role in pool sanitizing.











































