A recent article titled “Key Misunderstandings and Problems in Pool Water Chemistry” was intended to provide helpful information to pool operators, however, some of the chemistry referenced in the article is not completely accurate. It is understandable that there may be confusion within the industry and among pool operators, but misinterpreting the science may cause unexpected issues or put swimmers at risk. What follows will attempt to clarify some of the basic chemistry that was discussed in these concepts and perhaps give readers a better grasp of the science involved. References are provided for each concept so that the science can be independently verified if so desired.
Issue #1
As stated in the original article: “Liquid chlorine does not raise pH. When added to water, liquid chlorine (which has a pH of 13) makes HOCl (hypochlorous acid – the killing form of chlorine) and NaOH (sodium hydroxide), which raises pH. But when the HOCl is degraded by UV, and when used in killing and oxidation, it creates HCl (hydrochloric acid). The amount of HCl is almost identical to the amount of NaOH. So the net effect on pH is zero (or almost zero).”
It is well known that the equilibrium of hypochlorous acid in water is as follows:
HOCl ← → H+ + OCl-
It’s important to understand strong vs. weak acids and bases. A weak acid or base will only partially dissociate in water, while a strong acid or base will completely dissociate. Scientists use pKa (for acids) and pKb (for bases) values to interpret the strength and predict the dissociation. Hypochlorous acid (HOCl) is a weak acid and has a pKa of around 7.5. This means that at pH 7.5, the concentration ratio is about 50/50 reactants to products. Or to say it another way, it is about half dissociated at pH 7.5. Sodium hydroxide (NaOH) is a by-product of the addition of liquid chlorine. NaOH is a very strong base with a pKb value of 0.2. This very low value indicates that NaOH will be completely dissociated into Na+ and OH- in solution. By looking at these known constants, one can easily see that around pH 7.5 there will be about half the concentration of hydrogen ions as hydroxide ions because the HOCl does not completely dissociate. This causes an increase in pH. In theory, when liquid chlorine is added to water, you should get one hypochlorous acid for every one sodium hydroxide. This is an example of the basic chemical principle that a solution of a weak acid and strong base (in approximately the same concentration) will always yield a solution with a high pH. Conversely, a solution of a strong acid and weak base will yield a solution with a low pH. There are other constituents in swimming pool water that interact with the OH-, so it isn’t necessary to worry that the pool water will reach the extremely high level of a pure sodium hydroxide solution. The net effect, however, will be an increase in pH.
While the scenario above would be enough to create a high pH solution, in reality there is excess sodium hydroxide in liquid chlorine products. The excess NaOH is necessary to make the sodium hypochlorite and some remains after the process is complete. This excess NaOH is added to the pool water along with the liquid chlorine.
That’s still not the whole story, however. The strong base/weak acid scenario assumes solutions of distilled water with nothing to react with the HOCl. It is also well known that the disinfecting agent is HOCl and not the hypochlorite ion. Because this reaction is in equilibrium and is reversible, as HOCl is used up (either by oxidizing, killing microorganisms or sunlight degradation), then the hydrogen ion and hypochlorite ion will combine to form more HOCl. In chemistry, Le Chatelier's principle (also called The Equilibrium Law) states that when any system at equilibrium is subjected to change in concentration, temperature, volume, or pressure, then the system readjusts itself to counteract the effect of the applied change and a new equilibrium is established. In this instance, the “hypochlorous acid system” would be subjected to a change in concentration — as the HOCl is used up, then its concentration is reduced. In order to adjust the hydrogen and hypochlorite form more HOCl until it is all used up. There is no longer H+ in the water affecting the pH. This leaves an even greater excess of OH- ions than a weak acid/strong base in distilled water.
In addition, as the HOCl reacts with material such as organics, it does not generally form hydrogen ions. There is no excess H+, which would be needed in order to lower the pH and counteract the excess OH- added to the water as a by-product of liquid chlorine (or other hypochlorite).
It is important to understand that the use of liquid chlorine or other hypochlorites will raise the pH of water. These products are good disinfectants and the side effects can easily be managed, but it is important to understand them and make the appropriate adjustments.
Issue #2
“The free chlorine level should be 7.5 percent of the cyanuric acid level and cyanuric acid should not rise above 50 ppm.”
While it is true that much of the chlorine in the water may be bound to the cynauric acid molecule at any given time, the bond is extremely weak. Each trichlor has three available chlorine molecules that can be used to form hypochlorous acid, which means that there is a rate constant associated with each chlorine leaving and reattaching to the cynauric acid ring (trichlor to dichlor to monochlor to cyanuric acid). Those values are readily available. Scientists use rate constants in order to determine how quickly a reaction will happen. Rate constants can be compared between species in order to compare the strength of one chemical bond to another. Using those values and normalizing them to the rate constant of monochloramine, one can easily make a comparison about the strength/weakness of the trichlor bond and how easily they can be broken. These normalized values are given in the table below: 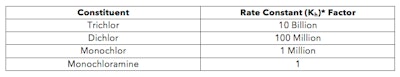
*Rates normalized by order of magnitude to Monochloramine; Data provided by Kirk-Othmer Encyclopedia of Chemical Technology
To interpret the table above, a chlorine will leave (or reattach to) the trichlor ring (to form hypochlorous acid) 10 billion times faster than chlorine will leave the monochloramine molecule. That’s extremely fast. To say it another way, the bond between chlorine and the cyanuric acid ring is 10 billion times weaker than the chlorine-nitrogen bond in monochloramine.
Another way to look at how quickly reactions occur is by going back to the basics, specifically the law of mass action. This law states that the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants. Simply stated, the higher the concentration of reactants, the faster the reaction will go. Because contaminants are a reactant in this chemical scenario, then the more contaminants that are in the water then the faster trichlor reacts to that (until you have used up all the trichlor and hypochlorous acid, of course).
Based on these basic chemical principles, one can easily see that it is unnecessary to raise chlorine based on cyanuric acid levels. The trichlor molecule is perfectly capable of reacting and adjusting to the conditions in the water.
While looking at the theory is interesting and helpful, what pool owners really care about is what happens in a real scenario. For that, we turn to a 1978 paper published in the Journal of Environmental Health (authors F.W. Linda and R.C. Hollenbach). This paper is particularly helpful because it combines the data from several field studies as well as taking into account some of the distilled water beaker studies. Note that most beaker studies are done in distilled water with no chlorine demand and are not always indicative of a real pool scenario. In fact, in 1963 a doctoral thesis was put together by J.R. Andersen at the University of Wisconsin which stated that, “these results were obtained under laboratory conditions and caution should be used if extended to actual swimming pool operation.” Much like the theory, it’s a good place to start but doesn’t always give the full picture. There have been a number of field studies, however, that provide much more applicable data. The conclusions reached in this peer reviewed published paper are as follows:
1. Cyanuric acid up to at least 200 ppm does not have a deleterious effect on the bactericidal efficiency of chlorine in swimming pools under actual field conditions. Indeed, it was seen to enhance disinfection in some cases.
2. Cyanuric acid significantly reduces the solar ultraviolet destruction of chlorine. Economies of up to 65 percent less chlorine usage were claimed. In addition, a free chlorine residual could be continuously maintained.
3. Chlorinated cyanurates are biocidally, at least, equivalent to inorganic hypochlorites or other accepted disinfectants. In some instances, superior performance was cited.
4. Cyanuric acid delays the bactericidal effect of chlorine in distilled water. The delay may increase progressively with higher cyanuric acid concentrations. This effect becomes insignificant in actual pool waters where longer kill times are observed with or without cyanuric acid.
5. The level of the free available chlorine residual is the primary factor in maintaining satisfactory disinfection. A minimum residual of 1ppm had been suggested.
6. Combined chlorine (chloramine) is an ineffective disinfectant, the presence of which in pool water must be minimized by periodic superchlorination.
7. The successful operation of a swimming pool requires the maintenance of proper ranges for such parameters as pH and alkalinity. If these parameters are not confined to the appropriate range, the biocidal efficacy may suffer.
8. In addition to proper chemical treatment, a good mechanical system is required, such as pump and filter. Maintenance on a continual schedule is also needed.
Note that the above list is taken directly from the paper. This paper cites twenty-three different peer-reviewed references in order to reach these conclusions.
Based on theoretical chemistry and peer-reviewed field data, it is very clear that cyanurates are a very viable and convenient option for pool water disinfection, the goal of which is to protect swimmers from disease transmission. In fact, data exists illustrating that levels up to 200ppm are acceptable, and there are significant benefits to having cyanuric acid in the water.
Additional data exists in support of chlorinated isocyanurates. In 1961 by Ditzel, et. al., reported that, “chlorinated isocyanurates do as effective a job of killing bacteria as sodium hypochlorite and that the addition of up to 100 ppm cyanuric acid did not decrease the bactericidal efficiency of available chlorine.” And in 1963, a study conducted by Gilcreas and Morgan observed no difference in the survival rate of E. coli and M. pyrogenes when exposed to levels of chlorine varying from 0.3-1.0 ppm, delivered by sodium hypochlorite and sodium dichloroisocyanurate.
To address the algae issue…the reason that some pools see algae growing in the presence of chlorine is that there are species resistant to chlorine. Algae have carotenoids which some species use as a defense mechanism. Carotenoids are anti-oxidants (or reducing agents) which means that they neutralize oxidizers (such as chlorine). When the chlorine comes in contact with one of these algae species, it is neutralized before it can kill the algae. Cyanuric acid has no bearing on this phenomena. In these instances, it is best to turn to another type of EPA-registered algaecide such as copper or a quaternary ammonium compound.
Conclusion
In conclusion, there are a number of EPA/PMRA-registered chlorinating products available for pool owners. Some of these may present additional challenges such as raising the calcium level or pH. Some may cause a decrease in pH. Again, most of these side issues can be easily managed with proper testing and maintenance. Always test and adjust pH after any product addition. Protecting swimmers from disease transmission should always be our top priority. To do that, follow directions on the product labels and maintain a chlorine residual within that recommended range (typically 1-4ppm).
Listed below are references used in support of the chemistry explained in this article. Readers are encouraged to consult these references, if desired. All journal articles listed in this reference section are from peer reviewed journals. (Note: "Peer review" means that a board of scholarly reviewers in the subject area of the journal review materials they publish for quality of research and adherence to editorial standards of the journal before articles are accepted for publication. If materials are used from peer-reviewed publications they have been vetted by scholars in that field for quality and importance.)
• Kowalski, X., Hilton, T.B., 1966. “Comparison of Chlorinated Cyanurates with Other Chlorine Disinfectants,” Public Health Reports, 81, pp 262-288.
• Ditzel, R.G., Matzer, E.A. and Symers, W.R. 1961, “New Data on the Chlorinated Cyanurates,” Swimming Pool Age 35, No. 10.
• Gilcreas, F.W. and Morgan, G.B. 1963, “Chlorinated Cyanurates and the Effect of Cyanuric Acid,” Swimming Pool Age, 37, No. 12, pp 30-38
• Andersen, J.R. 1963, “The Influence of Cyanuric Acid on the Bactericidal Effectiveness of Chlorine.” Ph.D. Thesis, University of Wisconsin.
• Weidenbach, K. N. 2004. “Swimming Pool Water Quality: An Analysis of Outdoor Public Swimming Pools in Pinellas County, Florida.” Master’s Thesis. Emory University.
• Linda, F.W., and Hollenbach, R.C. 1978, “The Bactericidal Efficacy of Cyanurates – A Review”, Journal of Environmental Health, Vol. 40, No. 6, pp 324-329
• Shriver, Atkins & Langford, Inorganic Chemistry, 2nd Edition
• Zumdahl, Steven S., Chemistry, 2nd Edition
• Carey, Francis A., Organic Chemistry, 2nd Edition
• Snoeyink and Jenkins, Water Chemistry, 1980
• Faust and Aly, Chemistry of Water Treatment, 1983
Karen Rigsby has a chemistry degree from Georgia Tech. She began her career as a forensic chemist with the Georgia State Crime Lab. She moved to the rec water industry in 2001. Rigsby is employed by BioLab, a chemical manufacturer whose specialty is trichlor and other ancillary products. BioLab’s parent company, KIK Custom Products, is a large bleach manufacturer. This diversity gives her special insight into the manufacture and end use of both isocyanurates and hypochlorite based products. Rigsby’s main focus has been education and new product development. She currently holds a patent for the development of a rapid chlorine demand test. She has served on APSP’s Recreational Water Quality Committee where she had a large part in writing water chemistry standards. Rigsby has recently become certified to teach science in the state of Georgia and is very enthusiastic about science education.