In order to produce the best pool and spa water we can, we use water balance indexes as tools to help predict whether water will deposit or dissolve calcium carbonate; or in other words, whether water tends toward the formation of scale, or tends to be aggressive.
These water balance indexes used by the swimming pool/spa industry are not guides for proper sanitizing or for human health and safety issues. Instead, they are an indication of calcium carbonate solubility and its effect on pool surfaces and equipment.
Maintaining proper pool/spa water balance (in addition to sanitizing) is important to help prevent calcium carbonate (scale) developing, or to prevent calcium from being removed (etched) from pool surfaces, particularly plaster/cement finishes.
RELATED: Keeping Pool Water Balanced
Saturation indexes include the Langelier Saturation Index, the Ryznar Stability Index and the Hamilton Index. This article addresses the scientific principles and formulas, limitations, application and similarities and differences between these Indexes.
LANGELIER SATURATION INDEX
The Langelier Saturation Index (LSI) was developed by W. F. Langelier in 1936 to predict the tendencies of municipal water to precipitate or dissolve calcium carbonate. The LSI is based on a theoretical principle taking into account the pH, temperature, total dissolved solids (TDS), bicarbonate and carbonate alkalinity and calcium hardness of a given water, and establishes the pH at which the saturation of calcium carbonate occurs (pHs). The LSI number is equal to the actual pH minus pHs.
Langelier's original formula was revised and modified by Larson and Buswell, and later by Van Waters and Rogers. The familiar equation used by the pool industry for years is as follows:
LSI = pH + CF + AF + TF – 12.1
[Where F stands for a "factor" based on the base 10 logarithm (sometimes slightly adjusted) of the calcium hardness (CF), carbonate-species alkalinity (AF) and temperature (TF), with 12.1 being a Log-derived "constant" based on a TDS value of approximately 500 ppm.]
The LSI formula has again been updated and improved by taking into account higher total dissolved solids content found in many swimming pools, and isolating carbonate alkalinity from cyanurate alkalinity (Wojtowicz, 1998). Today, this modified version of the LSI is used by some in the pool industry, often referred to as simply the Saturation Index (SI).
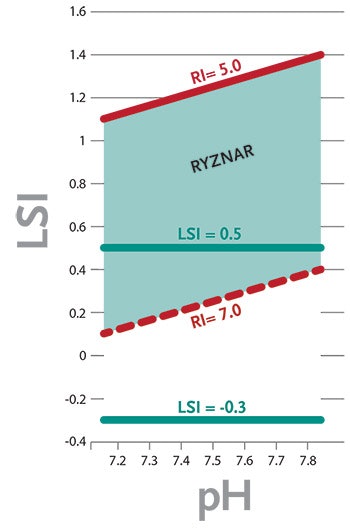 Figure 1. Comparison of Preferred Ranges in the Langelier and Ryznar Indexes - Click to enlarge
Figure 1. Comparison of Preferred Ranges in the Langelier and Ryznar Indexes - Click to enlarge
According to the LSI:
- When the LSI is zero, the water is balanced. Calcium carbonate scale should not form, nor should calcium carbonate be dissolved from cementitious surfaces.
- When the LSI is above zero, the water is potentially scale forming. The higher the number, the stronger the tendency is for calcium carbonate scale to form.
- When the LSI is below zero, the water is potentially aggressive towards calcium compounds in cementitious materials. The lower the number, the stronger the tendency is for etching to occur. Aggressive water may also be corrosive to metal surfaces.
The minimum and maximum LSI for proper pool water balance is considered by APSP to be -0.3 to +0.5. Within these parameters, water should be neither significantly aggressive nor scale forming. A positive value may be preferred over a negative value because a slight scale layer provides some protection, and is less harmful than corrosion, which causes permanent damage to mechanical and structural components.
RYZNAR STABILITY INDEX
The Ryznar Stability Index (RSI), developed by J. W. Ryznar in 1944, is based on the same water parameters and saturation of calcium calculation as used by the LSI. However, Ryznar made a mathematical adjustment to the LSI in order to always achieve a positive index number (RSI = 2pHs – pH). In addition, the RSI is mainly based on empirical observations of calcium scaling and the corrosion of metals in municipal water systems.
According to the RSI:
- Water is considered balanced and not corrosive nor excessively scale forming when the value is from 5.0 to 7.0. (Various targets are cited. Consult equipment manufacturer literature for recommendations.)
- When the RSI is above 7.0, the water is considered to be potentially corrosive.
- When the RSI is below 5.0, the water is considered to be potentially scale forming.
A comparison between the RSI and LSI for typical swimming pool/spa water parameters shows that maintaining a balanced RSI of 5.0 to 7.0 roughly corresponds to maintaining an LSI of about +0.1 to +1.4. (See chart.) Some heater and equipment manufacturers prefer the RSI because it provides greater protection against corrosion of metals.
HAMILTON INDEX
The Hamilton Index (HI) is actually a chart developed by experienced service tech Jock Hamilton through field service and research over a period of 11 years beginning in the 1960's.
There are several principles behind the concept of the HI. It somewhat correlates to the LSI, however, it has its own unique differences and recommendations. First, the HI chart recommends maintaining the pH between 7.8 and 8.2. Second, depending on the total hardness (not calcium) level, the total (not carbonate) alkalinity is then adjusted to a target level. The higher the total hardness of the water, the lower the recommended or target total alkalinity. The HI chart does not consider TDS, cyanurate content or temperature.
LIMITATIONS AND WHAT THESE INDEXES DO NOT PREDICT
It should be understood that these indexes, including the LSI, RSI, and HI, do not necessarily predict the corrosion of steel and other metals. Corrosion of metals is generally a function of pH and the concentration of anions (e.g. sulfates and chlorides) and oxidants, which would need to be taken into account. There are corrosion indexes, such as the Riddick, Larson-Skold, Stiff-Davis, Casil, and Pisigan & Singley Indexes that are more applicable to metal corrosion issues. It is possible for non-scaling (aggressive) water to also be non-corrosive to metals. Conversely, it is possible for "scaling" water to also be "corrosive" to metals. Therefore, the saturation indexes are not completely reliable with respect to corrosion of metals.
RELATED: The Four Habits of Highly Effective Water Analysts
These indexes will predict active tendencies, and even the relative strength of these tendencies, but it will not predict HOW MUCH calcium carbonate or other calcium species will be deposited or dissolved.
Water can be balanced, and therefore not aggressive to calcium carbonate, even when individual parameters are well outside minimum or maximum recommendations. For example, water with a 7.8 pH and a 180 ppm carbonate alkalinity can be balanced with about 100 ppm calcium hardness (contingent on TDS and temperature).
It is important to note that even when water parameters are within the recommended ideal ranges, the water can still be outside the recommended LSI range of -0.3 to +0.5. For example, water with a pH of 7.4, a carbonate alkalinity of 80 ppm, and calcium hardness level of 200 ppm, but with an elevated TDS of 3,000 ppm or with cold water temperature below 60 degrees can result in an LSI of below -0.3.
Since water parameters are constantly changing, there is no steady state in saturation chemistry. Rather, the indexes are predictive tools used to anticipate general tendencies in the one direction or the other. Water can have a very high LSI and still not experience calcium carbonate scaling. Water that contains sequestering agents can inhibit scaling tendencies. Additionally, these indexes are not designed to predict the potential of calcium sulfate scaling, which also can affect the solubility of calcium carbonate. Testing accuracy is important to obtain an accurate index number.
An index result is a "snapshot" of the characteristics of the water at the time the sample was obtained, not necessarily an indication of what has happened in the past or what will happen in the future. In fact, when looking at pool water after a scaling or aggressive event, it should be understood that once precipitation (deposition) or dissolution (etching) of calcium carbonate occurs, the LSI changes. When precipitation of calcium carbonate occurs, the index number lowers; when dissolution of calcium carbonate occurs, the index number rises.
There is some confusion regarding balanced water with the Ryznar Index. While it appears that water with a Ryznar Index of 5.0 to 7.0 has been established as balanced, Ryznar himself stated that an index from 7.0 to 7.5 may not form a protective layer of calcium carbonate on piping, indicating that in some cases, no scaling or corrosion may occur when the water is between these two values.
In this regard, it appears that the RSI focuses more on preventing corrosion of metal and piping. Water that does not form scale can be corrosive to metal, which may explain why that index is shifted toward preferring slightly scale-forming water (as established by the LSI). Laying down a thin layer of scale seeks to ensure that no corrosion of metal occurs.
RELATED: Water Chemistry: Lessons Learned
Common industry errors made when using the LSI or the RSI when calculating calcium saturation include the use of total hardness in place of calcium hardness, the use of total alkalinity instead of carbonate-species alkalinity and the use of 12.1 as a constant rather than as a TDS variable. This last error is especially apparent in salt pools where TDS levels far exceed the 500 ppm represented by the 12.1 value. The Hamilton Index uses TOTAL hardness and TOTAL Alkalinity.











































