
QUICK OVERVIEW
A few quick facts about boric acid and sodium borates:
- Used as a pH buffer in swimming pools (against increases in pH)
- Helps to limit algae growth (acts as an algaestat)
- Lowers chlorine demand
- Additional benefits may include reduced corrosion
- Recommended dose range of 30 – 50 ppm (as boron)
- Dosing is dependent on the form of boron used (e.g. boric acid or borax)
Boric acid and sodium borates are commonly used as a pH buffer in swimming pools and spas, meaning they help increase the capacity of the water to resist changes in pH. However, they have other uses as well: Boric acid and sodium borates can inhibit algae growth and reduce corrosion. Some even say they improve the "feel" of the water by adding a tint or sparkle.
A recent review of the use of boric acid and borates in swimming pools found supportive evidence for the claims of reduced corrosion; however, more scientific evidence is needed to support claims that boric acid contributes to tint, sparkle and softer "feel."
Regarding algaestatic effects, the review noted there was no scientific support for the common assertion that borates starve algae of CO2 and that the algaestatic activity is more likely to involve multiple molecular targets given the affinity of boric acid to form complexes with poly-hydroxyl compounds.
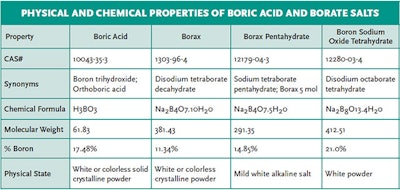
Boric acid is the predominant form at the typical pH range in swimming pools and spas (pH 7.2-7.8). Sodium borate salts (such as borax) can also be utilized as substitutes for boric acid and have similar properties and functions. The physical and chemical properties of the boric acid and its borate salts are shown in the table below:
APPLICATION
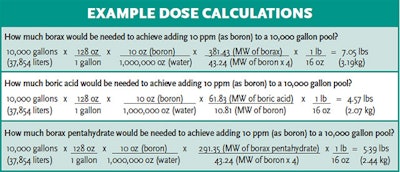
Boric acid and sodium borates are typically sold in white crystalline powder form. The powder is typically broadcast into the water via scoop or plastic bowl while walking around the pool's perimeter. In order to achieve the intended functions of borates, a common dose is 30-50 ppm as boron but 50 ppm should not be exceeded. While typically described as 30-50 ppm borates, borates are measured in ppm of boron. A pool operator must be careful to calculate the amount of boric acid or sodium borate salt necessary to obtain the correct concentration in the pool (as ppm boron). A few example calculations are provided below.
It is important to recognize that borax products will raise the pH of the water when added. Therefore, it is necessary to check for and remove excess metals in the water (copper or iron) to reduce the possibility of staining.
RELATED: Using Air and Acid to Quickly Get Perfect pH/Alkalinity
Following addition of sodium borates (e.g. borax), the pH of the swimming pool will need to be lowered through the addition of muriatic acid or a dry acid such as sodium bisulfate. The amount of acid required will be dependent on the type and amount of borate used.
Not to be confused, boric acid is a weak acid that will only lower the pH slightly following addition. Use of boric acid does not require further addition of muriatic acid or removal of excess metals. Product labels will provide instructions for calculating the appropriate amounts of boric acid or sodium borates for a given swimming pool or spa volume.
As with any other pool chemical parameter, monitoring the concentration of boron should be included in routine testing of the pool or spa water. Boron levels can be reduced due to water loss (backwashing, filter cleaning, splash out or drag out). When boron concentrations fall below 30 ppm, sufficient boron should be added to restore the concentration to 30-50 ppm. There are several commercially available borate test strips and testing devices. Although these are labeled as "borate" tests, they measure boron concentration.
PRECAUTIONS
In order to safely use and handle boric acid and sodium borate products, all individuals involved in their production, distribution, sale and use should be trained and knowledgeable about their properties. Safety information is available on the product label, product safety data sheets and manufacturer's training materials. This safety information will include product and packaging disposal instructions and spill response information.
The available toxicity data for boron (as boric acid/borate salts) indicates moderate acute toxicity by the oral route of exposure. Cases of accidental boron poisoning have been reported indicating that the lethal doses of boron range from 15-20 grams for adults, 5-6 grams for children and 2-3 grams for infants.
RELATED: Borates for the SCG
The systemic toxicity of boric acid and sodium borates has been assessed by various agencies and organizations, including the EPA, which has set safety limits for the oral exposure to boron based on toxicity data from repeated dose studies in animals. Reproductive and developmental toxicity findings were identified as the most sensitive effects in animals following repeated oral exposure to boron. The U.S. EPA has set 0.2 mg/kg-day as an acceptable oral intake of boron from all sources which equates to 14 mg/day (based on a 70 kg body weight). Assessment of boron by the U.S. EPA and NSF International found that the currently recommended boron concentrations in swimming pools or spas of 30-50 mg/L as boron do not pose a significant risk to human health.
It is important to note that the above application rates have been set based on health protective concentrations; therefore, the swimming pool operators for pools using boric acid or sodium borates should regularly verify that appropriate concentrations are maintained.











































