Treating pool and spa water can be a surprisingly multi-faceted proposition. While most people in our industry understand the basics of oxidation, sanitizing and water balance, there are aspects of water chemistry that confound even the most experienced practitioners.
In particular, the way that ammonia works in pools and spas, from its positive applications to how it results in disinfection byproducts and the formation of nitrates — one of the most problematic common compounds in water — is a source of mystery and misconception for many.
To help foster better understanding of these key chemistry issues, here's a breakdown of the science behind ammonia and nitrate as they relate to recreational water treatment.
MORE THAN A CLEANER
Ammonia is a colorless, strongly alkaline gas with a pungent odor that is seemingly everywhere in our physical universe. It was the first complex molecule discovered in space in the galactic dust clouds of the Milky Way, and it also makes up the rings of Saturn. Closer to home, volcanoes and hot springs were the original early sources of ammonia on our planet.
Ammonia takes its name from an Egyptian deity Ammon because a very early discovery of ammonium salts were found in camel dung near the ancient site of his temple. As a gas, ammonia consists of one nitrogen atom and three hydrogen atoms (NH3). It is very soluble when mixed in water, where it creates an alkaline liquid known as ammonium hydroxide.
RELATED: New Thinking: Chlorine/Cyanuric Acid In Balance
Liquid ammonium hydroxide is the proper name for regular household ammonia used for disinfecting and cleaning. As an industrial chemical, ammonia is used in a variety of common products including fertilizer, nitric acid, nylon, explosives and countless others.
Ammonia enters the atmosphere from agriculture, autos and industrial processes, as well as from livestock manure and agricultural fertilizer. When ammonia gas mixes with atmospheric emissions, it creates microscopic particles. These particles measure about 2.5 micron in size, or about 1/3 the width of a human hair. Particle excess is deposited back to Earth primarily through rain precipitation.
INTO THE POOL
When used in well-maintained pools along with recommended levels of free chlorine, ammonia will combine to form inorganic chloramines. During this process, ammonia releases nitrogen, which converts to nitrite, which then converts to nitrate. (Which explains why nitrate levels rise in pools after heavy rains.) High nitrates levels will increase the consumption of free available chlorine and cause excess algae growth. (We'll return to nitrates later.)
What most people don't realize is the key role ammonia plays in creating some of most commonly used chemical products. For example, saturating salt with ammonia and carbon dioxide creates sodium bicarbonate. When heated, sodium bicarbonate creates soda ash. Other examples include quaternary ammonium and poly-quaternary ammonia, which are used to fight green algae. Both of these products incorporate an ammoniated compound that serves to lower the water surface tension. This causes the algae to take in a toxin, splitting the cell wall and killing it.
Some in the pool industry believe the use of ammonia-based products is problematic because of the erroneous belief that these products will leave ammonia in the water. But nothing could be further from the truth. Is there ammonia left behind in the pool from use of an ammonium salt? Would even adding straight ammonium hydroxide result in ammonia in the pool? The answer is no because the chemical reaction in swimming pool water ammonia quickly combines with chlorine to form inorganic chloramines.
FAST REACTIONS
Products that contain ammonia are not the problem in pools. The fact is, ammonia technically does not exist in pool water due to the quick chemical reactions that take place, especially with chlorine.
As soon as ammonia enters a chlorinated pool, it reacts with hydrogen ions to quickly form ammonium ion. The ammonium ion reacts with hypochlorous acid to form monochloramines. Monochloramine reacts with free chlorine to form dichloramines, which are unstable and rapidly decompose.
From that point, its nitrogen content oxidizes into elemental nitrogen gas and releases into the atmosphere. Chlorine shock oxidizes the ammonium and free chlorine combines to create monochloramines.
There are two kinds of chloramines present in swimming pool water: inorganic chloramines, which equal chlorine combined with nitrogenous ammonia; and organic chloramines, which equal chlorine combined with organic nitrogen waste. Inorganic chloramines are the result of the quick reaction of chlorine with ammonia.
Because inorganic chloramines hold together longer than free chlorine, many drinking water facilities use chlorine and chemical ammonia to produce chloramines for disinfecting. These inorganic chloramines are more stable and maintain a residual longer than free chlorine. They will last from the water station through the pipes and to the faucet. This causes a strong chloramine smell at the tap. (This manufactured form of disinfecting drinking water is known as chloramination.)
If you source water utilizing this method, it's critical to test the water to determine the level of chloramines. Once the level is determined, increasing the free chlorine level can help to reduce the added chloramines. Organic chloramines from chlorine combined with organic nitrogen are more difficult to break apart then the inorganic chloramines because hey do not respond to breakpoint chlorination. In fact, it can take many frequent superchlorination shocks to begin to see any reduction.
Another method could be frequent oxidizing with a non-chlorine shock known as potassium monopersulfate. The easiest and most efficient way to remove organic bound chloramines is by draining and dilution.
One might wonder how to tell the difference between inorganic-bound chloramines or organic bound chloramines since a test cannot determine which is which. To do this, re-test the total and free chlorine again after performing additional chlorination or raising free chlorine. Any combined numbers that remain after this are organic chloramines.
Draining, dilution and additional shocking will remove the organic chloramines. Ozone units or UV are two proactive ways to prevent the build-up of combined chloramines in either the inorganic or the organic form. Ozone is a stronger oxidizer than chlorine and can break apart and prevent the build-up of both inorganic and organic chloramines, while UV systems act to oxidize the precursors to chloramines. Both ozone and UV act as secondary disinfectant systems according to the Model Aquatic Health Code.
THE NITROGEN CYCLE
Atmospheric nitrogen from storms converts to nitrates in pools via a complex set of reactions known as the "nitrogen cycle."
Waste from swimmer perspiration and ure athe primary sources of detrimental nitrates. Human adults secrete up to an ounce of dilute urea daily in their urine, and urea contributes 85 percent of total nitrogen, making it an ideal nutrient for bacteria and algae.
RELATED: Is Superchlorinating The Best Way To Eliminate Chloramines?
Urea is also a primary source of ammoniated chloramines. An rare result of urea and chlorine will be the formation of toxic chloramines such as cyanogen chloride (CNCl) and trichloramine (NCl3). These are both very toxic to breathe and therefore especially problematic in indoor pools and can cause lung and eye irritation to swimmers in facilities that lack proper ventilation or effective water treatment.
We know that peeing in the pool is never a good thing. However, urea is also present in sweat, and active swimmers can exude a pint of perspiration per hour. We may be able to stop the pee, but we cannot stop the sweat.
Nitrates can also be introduced to the pool from animals, from pet dogs and wild ducks to bears. Improper maintenance and lack of oxidation will lead to high chloramine levels.
Poor disinfection, algae or lots of organic debris will increase nitrates. Replication of the nitrogen cycle takes place in poorly maintained pools by certain nitrifying bacteria like pseudomonas. Pollen can distribute nitrogen into pools as well. Excessive blue-green algae in pools contains cyanobacteria, which is also nitrogen fixing. Green pools take more nitrogen from the atmosphere and nitrification leads to more nitrates. In farm areas, nitrates are present in the groundwater.
The recommended maximum level for nitrates in drinking water is 10 ppm. This applies to swimming pools as well, although some states allow 10-25 ppm is allowable. High levels of nitrates do not pose a health threat to adults; however, infants or toddlers that consume nitrates can develop "blue baby syndrome," a condition that causes a lack of sufficient oxygen to the red blood cells.
NITRATE EFFECTS
The most abundant form of nitrogen in pools is not from ammonia but from nitrates. Ninety percent of ammonia in pools oxidizes to nitrogen and then is released back into the atmosphere. Heavy swimmer loads with excessive waste, large amounts of organic debris and algae in pools are the primary culprits to high nitrate levels.
RELATED: The Science of Starving Algae
In short, a well-maintained pool free of organic debris with routinely performed practical oxidation will not experience problems from nitrates. While storms bring in nitrates, immediately shocking afterwards can help to keep them at bay. Promptly dealing with algae also helps reduce the mechanism for the formation of nitrates. Nitrates at levels above 25 ppm can lead to rapid decomposition of free chlorine and excessive algae problems.
USEFUL CONCLUSIONS
Obviously, there's a lot to keep in mind when it comes to understanding and managing ammonia and nitrates in pool and spa water. For clarity, here's just a few conclusions you can take away from this story:
- Ammonia does not exist in pool water because it immediately reacts with hydrogen ions to form ammonium ions
- Ammonium ions oxidize rapidly by proper free available chlorine
- Ammonia-based specialty chemicals will not cause poor water quality
- Organic nitrogen compounds (organic chloramines) are the real problem in pools because they oxidize at a much slower rate than ammonia-bound chloramines (inorganic chloramines)
- Ammonia contributes less than 10 percent of nitrates in pools.
- Poorly maintained pools with persistent organic debris and algae will cause increased nitrates. Pets and wild animals can bring nitrates in through waste
- Nitrates can increase in pools immediately after a storm
- Urine and perspiration in reaction with insufficient free available chlorine are primarily responsible for toxic chloramines and increased nitrates
- Proactive oxidation and proper maintenance can help prevent the increase of nitrates
- Always test groundwater in agricultural areas for nitrates before filling pools
BASIC CHEMISTRY
When ammonia enters water three types of inorganic chloramines reactions can occur:
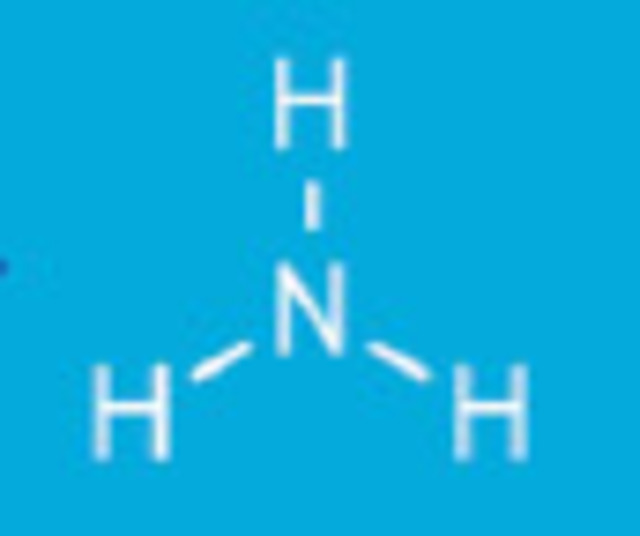
Ammonia is one nitrogen atom and three hydrogen atoms NH3.
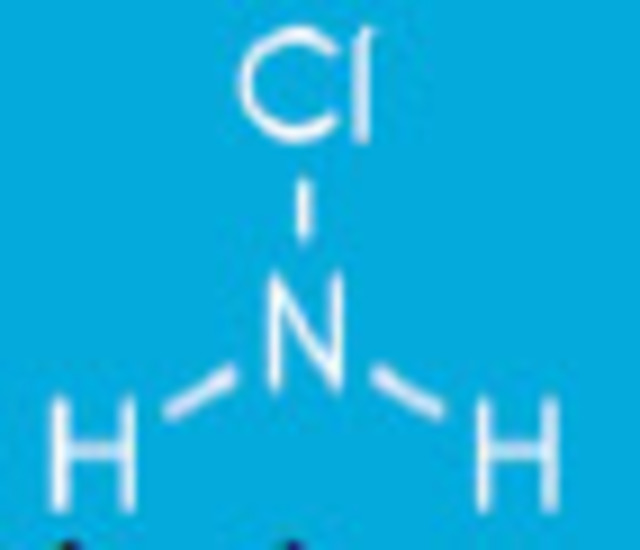
Monochloramines form when the ammonia molecule gives up one hydrogen atom for one chlorine atom. NH2Cl + H2O.
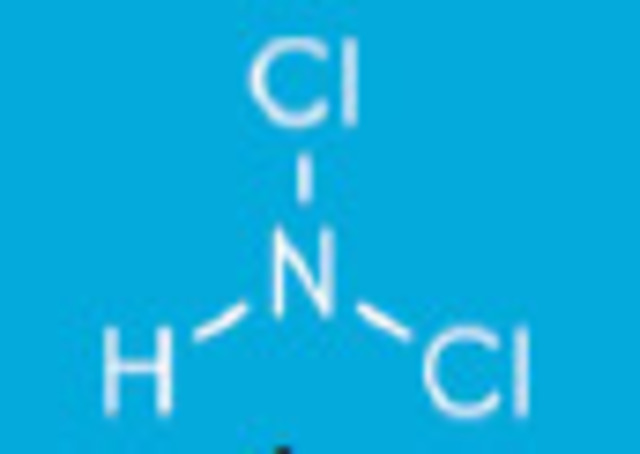
Dichloramines form when ammonia gives up two hydrogen atoms for two chlorine atoms NHCl2 + H2O
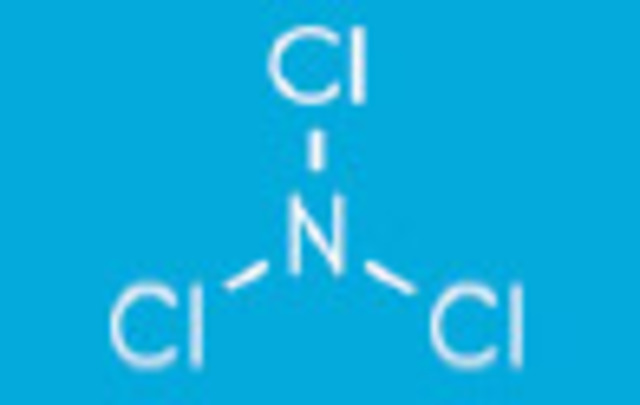
Trichloramines form when ammonia gives up all three hydrogen atoms for chlorine atoms NCl3 + H2O
Monochloramines can lead to some minor eye irritation to swimmers. Dichloramines will cause eye irritation and heavy chlorine odor in the air. Trichloramines are very harmful. Also known as tri-halo methane or nitrogen trichloride, they can cause severe lung and breathing problems and are carcinogenic. Nitrogen trichloride is formed when swimmers pee or perspire in the pool. The proactive practice of proper, regular oxidation can prevent the accumulation of detrimental chloramines, MPS, ozone or UV systems can help to prevent the build-up of harmful chloramines.











































