In recent decades, drought has become a more pressing issue in areas of North America, and along with it, water conservation methods for leisure aquatic vessels. One focus of conservation efforts has been reducing the necessity of draining and refilling in order to maintain healthy water.
Ideally recreational water should be replaced routinely based on the number of bathers or increase in measured total dissolved solids (TDS) levels. Under conditions of extreme or extended drought, however, strict conservation measures may prohibit draining and replacement of pool water. In such cases of continued influx of bather waste, natural organic matter, hardness ions, various salts, etc. — and no outlet via water exchange — a wide range of water parameters can climb to over recommended limits.
This article provides guidance for pool operators coping with these issues while trying to maintain safety for bathers and the integrity of pool surfaces and equipment.
CHALLENGES WITH WATER TESTING
A. OUT-OF-RANGE PARAMETERS
One problem with aged water is that some of the parameters may climb to values outside the accurate ranges of a typical test kit. If any test is at the lowest or highest end of the limits of that test, the actual result may be far different. For example, many cyanuric acid (CYA) test kits using a target detection method, do not provide accurate results for water containing more than 100 parts per million (ppm) CYA. The use of a photometer can extend the range up to 150 ppm. There are also test strips that read up to 250 or 500 ppm. These can be used as a screening test for very high concentrations of CYA. If a test with undiluted pool water gives a result at or near the limit of the kit (e.g. within the upper 20% of the range), the actual value may be far higher than the test results indicate.
In such a case, it is wise to dilute the pool water sample according to the manufacturer and test the diluted sample. The test result of the diluted sample should be multiplied by the dilution factor to get the actual concentration. For instance, if one part pool water sample is diluted with 3 parts distilled water, then multiply the result by 4.
For pH, color-based kits can also be a problem. Sample dilution does not work with pH. Use a pH meter to obtain an accurate reading.
B. TEST INTERFERENCES
Another issue is test interferences. If the parameter is within the range, other species in the water may have climbed to levels that cause an interference. The most common interference in pH and total alkalinity testing is high chlorine. Do not test pH after adding a chlorine shock. Very high chlorine causes a chlorine test to read very low. Non-chlorine oxidizers cause false high readings in DPD total chlorine tests. CYA and some algaecides cause alkalinity readings to increase. Copper and other metals interfere in hardness tests. High temperatures cause CYA test to read too low. Lower temperatures cause it to read high.
KEY WATER BALANCE PARAMETERS: CALCIUM HARDNESS, ALKALINITY and pH
A. CALCIUM HARDNESS
According to the ANSI/APSP-11 Standard, a calcium hardness range of 150 to 1,000 ppm is considered acceptable for pools. Under conditions of extended drought, calcium levels may drift higher. The pH and alkalinity can be reduced to offset the impact of the high hardness. Care should be taken to ensure that the alkalinity and pH do not go lower than recommended. This could lead to possible corrosion and scale issues. Hot surfaces in heaters or heat exchangers are likely places to anticipate problems. Water lines are another location where scale could form.
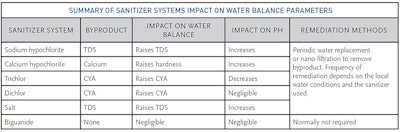
Calcium hardness is reduced by draining and replacing an appropriate amount of water to lower hardness. Replenishment of water after filter backwashing may be sufficient, but under severe restrictions regular filter maintenance may not be allowed. The water may self-correct by depositing scale until balance is restored. To prevent increased calcium in the water, calcium hypochlorite is not recommended. Dichlor, trichlor, and sodium hypochlorite do not contain calcium and can be used rather than calcium hypochlorite. The byproduct of calcium hypochlorite is calcium. Depending on the strength of the calcium hypochlorite, it may add 0.8 ppm for every 1 ppm FAC added in 10,000 gallons. Dichlor, trichlor or sodium hypochlorite may be preferred sanitizers to prevent increased calcium.
B. ALKALINITY
Alkalinity can be reduced by addition of acid. Keeping alkalinity at the low end of the acceptable range (60 to 180 ppm) can compensate for the gradual accumulation of calcium hardness in water balance (see Section d). Keeping bicarbonate alkalinity low has the fringe benefit of slowing pH increase from normal carbon dioxide loss to the atmosphere.
c. pH
The loss of carbon dioxide to the atmosphere causes the pH of water to rise unless acid is added to the pool. Addition of most acids will result in a gradual increase in TDS, resulting in increased corrosivity of the water. An acceptable pH range is 7.2 to 7.8.
D. WATER BALANCE CALCULATION
Balancing the pool water using the Langelier Saturation Index (LSI) is especially critical for drought conditions. To compensate for high TDS levels, test for and use the revised TDS correction factors recommended by PHTA for the LSI calculation. Water balance calculations are only as good as the data used. Alkalinity is one of the three most critical factors in determining LSI. Low alkalinity with moderate to high CYA concentrations may be a significant fraction of the alkalinity test result. Any errors in measuring either the total alkalinity or the CYA will significantly impact LSI calculations. Testing errors or interferences will compromise the water balance calculation.
DISINFECTION BY-PRODUCTS, TOTAL DISSOLVED SOLIDS, AND TOTAL SUSPENDED SOLIDS
A. DISINFECTION BY-PRODUCTS
Chlorine and bromine disinfectants react with microorganisms and other substances in the water to form disinfection by-products (DBP). DBPs are categorized into three major classes: inorganic by-products, organic oxidation by-products, and halogenated organic by-products. While thousands of DBPs are known, many have not been fully identified. Common DBPs include trihalomethanes (THMs), haloacetic acids (HAAs), chlorates and bromates. Many factors, including type of organic matter, the type and concentration of oxidizing disinfectant, the presence of other inorganic ions, temperature, and pH affect the composition of DBPs. Elevated concentrations of halogenated DBPs have been associated with increased health risks. It is important to prevent their accumulation.
B. TOTAL DISSOLVED SOLIDS AND TOTAL SUSPENDED SOLIDS (TSS)
TDS is the sum of dissolved organic carbon and dissolved inorganic salts. TSS are insoluble organic and inorganic matter suspended in the pool water. Disinfectants react with both TDS and TSS creating disinfection by-products. Elevated levels of TDS adversely impact bather safety by increased concentrations of DBPs. Degradation of equipment can occur due to the likelihood of galvanic corrosion. Elevated levels of TSS impacts bather safety by increased water cloudiness (turbidity) preventing visualization of bathers.
TDS is most commonly measured by use of a conductivity meter. Calibrated meters read direct TDS, using assumed proportionality between TDS and conductivity.
In drought-stricken areas, the 1,500 ppm TDS increase limit may be questioned during drought restrictions. Pool water may remain safe for swimming even if the TDS exceeds recommended limits. Pool operators should remain aware of large increases in TDS. This may mean the water has been over-used without normal replacement. Under such circumstances, other indicators of water quality should be monitored: odor, irritation to eyes, discoloration, etc. Increased concentrations of residual combined chlorine are a practical indicator of recreational water quality.
In this context, nuisance residual combined chlorine is not eliminated by breakpoint chlorination or oxidation. Combined chlorine levels that cannot be reduced below 0.4 ppm, along with irritable chloramine odors are clear indicators that water quality has degraded. There is no indicator for brominated recreational water. Bromamines dissociate too readily to be measured by common testing methods. One can slow the buildup of TDS by using calcium hypochlorite, dichlor or trichlor. Sodium hypochlorite contributes a greater amount of TDS to the pool in the form of sodium chloride.
Operators should be aware of the contribution to TDS from the sanitizers they choose.
CYANURIC ACID (CHLORINE STABILIZER)
Cyanuric acid stabilizes chlorine in the presence of sunlight. The APSP11 Standard sets a maximum limit of 100 ppm cyanuric acid in the water (ideal range between 30-50 ppm). High concentrations of cyanuric acid contribute to alkalinity and must be corrected for when performing water balance calculations. High cyanuric acid concentrations should be reduced by draining and replacing water.
To prevent increased cyanuric acid in the water, sodium hypochlorite or calcium hypochlorite can be used, rather than chlorinated isocyanurates such as trichlor (trichloro-s-triazinetrione) or dichlor (sodium dichloro-s-triazinetrione). Trichlor adds 0.6 ppm of cyanuric acid per ppm of chlorine and dichlor adds 0.9 ppm per ppm of chlorine.
ALTERNATIVES
If you are unable to replace water in a pool, the following options may be considered.
A. MEMBRANE FILTRATION
Membranes remove inorganic compounds, organic compounds, and particulate matter by creating a barrier through which water is preferentially passed. While both reverse osmosis (RO) and nanofiltration (NF) membranes are able to filter out both DBP precursors and DBPs, the increased flow rates and decreased pressures associated with NF membranes make them more economical than RO.
Hollow Fiber membrane filters are the most commonly used. These filters can reject particles, not by physical size, but instead by molecular weight. The greatest limitation of membrane technology is fouling of the membrane and loss of efficiency from the binding of microorganisms, colloidal or particulate matter, dissolved organic and slightly soluble inorganic compounds. Prefiltering solves this problem and is used to remove these contaminates.
Because particulates and colloids are major foulants, membrane feed waters with turbidities less than 1 NTU are recommended. It is important to note that RO and NF are less than 100% efficient. With common RO systems, 20–25% of the treated water is lost as a “retentate”, in which impurities are concentrated. This water would need to be discharged, with possible legal hurdles. Also, a comparable amount of makeup water would have to be added to the pool.
Studies show NF membranes remove 66% to 97% of trihalomethane, haloacetic acid, and other DBP precursors from pre-chlorinated water. There are fewer reports evaluating the performance of RO or NF technology with chlorinated pool water. One membrane system was evaluated that required chlorine levels not exceed 1.0 ppm; reduced calcium, sodium, chloride, sulfates, carbonates, heavy metals, cyanuric acid; and dissolved organic compounds. Salinity and calcium levels were reduced by 1000 and 500 ppm, respectively, in a 20,000 gallon pool after two days. Water loss was approximately 15% (3,000 gallons). In a second study, a lab-scale system attached to a sand filter for several weeks, with a chlorine-tolerant membrane, removed 70% to 80% dissolved organic carbon and absorbable organic halogens from pool water with slightly higher chlorine levels.
Many DBPs are so small they may not be removed by membrane filtration. Also, free chlorine attacks the membrane material and failure rapidly occurs for chlorine-sensitive thin-film membranes.
B. COAGULATION
Coagulation may help the control of TSS. Use of common coagulants like alum and cationic polyelectrolytes (polyamines) adds to TDS. Efficacy is pH dependent. Coagulants are typically applied in three ways:
1. Added directly to the pool water and removed by the filter.
2. Added directly to the pool water, settled to the bottom and vacuumed.
3. Added upstream of the filter as a filter aid.
No reported studies show effective coagulation reduction of TDS in chlorinated or brominated pool water.
REDUCING DBP PRECURSORS BY LIMITING CONTAMINATION
The best approach to minimize DBP formation is proactive reduction of DPB precursors. Controlling the entrance of natural and cosmetic organic materials in pool water should be incorporated. Bathers should shower immediately before entry into the water to remove suntan lotions, perspiration, body oils, and other organic contaminants. Especially for small children, bathroom breaks should be encouraged and urination in the pool should be discouraged. Leaves, grass, seeds, and other natural vegetation should be controlled by using screen rooms or temporary pool covers. Run-off from lawns and plant beds should be avoided.
A. REDUCTION OF TDS BY REDUCED CHEMICAL USAGE
TDS increases with every addition of pool chemicals. TDS levels can be managed by the appropriate and conservative use of additional chemicals. Excessive amounts of acid, base, and disinfectant should be avoided. Water soluble clarifiers and flocculants should be used only when better filtration methods are not available.
Copper and silver are effective against algae and bacteria; however, their use increases TDS. A monthly 10 ppm dose of sequestering agent adds dissolved organic carbon and disinfection byproduct precursors to the water. This increases TDS by 120 ppm annually. If metal ionizer systems or metal-based algaecides are used, insoluble metal adsorption polymers added to the skimmer could be better at preventing metal staining.
B. POOL COVER
Pool covers reduce the evaporative loss of pool water. However, they hinder the removal of volatile disinfection byproducts. The buildup of combined chlorine and other disinfection byproducts tends to be more severe and noticeable at indoor pools and spas than at outdoor venues. Indoor pool water was observed to be about five times as toxic as outdoor pool water in one study. Welldesigned ventilation systems that sweep volatile DBPs from the surface of the water and vent them to the outdoors can be critical to maintaining air and water quality at indoor pools. By limiting air exchange with the water solid pool covers increase the accumulation of disinfection byproducts.
Although not always practical for all pool applications, the addition of a pool cover conserves water by reducing the amount of make-up water needed by up to 50%. Additional benefits include reduction in pool chemical consumption by up to 60%, and up to 70% savings on heating costs.
PRECAUTIONS
Prohibition of normal water replacement in pools due to drought restrictions affects water quality in several ways:
- Filter backwash restrictions lead to increased filter pressure and decreased circulation. Reduced circulation leads to slower distribution of chemicals.
- Reduced sanitizer efficiency leads to an increase in potential recreational water illness RWI’s.
- Reduced oxidation, cloudy water, and algae lead to unsafe conditions for swimmers.
- Increased risk of microbial infestation and algae from accumulation of nutrient contamination.
- Irritating and toxic water from the accumulation of DBPs.
The net effect of poor water quality is the need to drain and dilute some or all of the pool.
Compared to other common water uses, the minimal water usage for normal pool maintenance is not extravagant. The value of aquatic recreation and maintaining healthy water are both very important. Therefore, regular filter backwashing and contaminant controlled are well warranted. There are alternate opportunities for saving water, including installation of high efficiency toilets, shower heads and washing machines; eliminating water for grass and gardens by changing to self-sustaining climate-appropriate landscaping; and reducing evaporative waste of water for crops by more efficient irrigation methods. PHTA and its affiliates have worked with various state and local chapters to educate government officials on the value of recreational water and the relative efficiency of this water use, and the importance of maintaining good water quality in recreational water. Working with such organizations to inform and influence regulators is preferable to accepting ill-advised sacrifices in water quality and safety.
CONCLUSION
Use and maintenance of swimming pools without adequate water exchange results in increased concentrations of DBPs, TDS and TSS. The efficient way to reduce the concentration of TDS is to drain and dilute some or all of the water. Due to drought restrictions this option may not be available. Membrane-based filtration methods may lower DPBs, TDS and TSS, but chlorine and high TSS reduce the efficiency of these methods, and some wastewater is still produced and must be discarded. While coagulation methods reduce TSS, they have not been effective in reducing DBPs and TDS in chlorinated pool water. The increase in DBPs, TDS, and TSS can be minimized by reducing the entry of contaminants and the moderate and appropriate use of pool chemicals.
REFERENCES
- Flaccus, Gillian, “Swimming pool industry touts itself as water-neutral option in California’s 4-year drought”, U.S. News & World Report, © 2015 Associated Press, June 1, 2015. http:// www.usnews.com/news/us/articles/2015/06/01/ pool-industry-touts-water-savings-incalifornia-drought
- See for example: “Water Balance for Pools & Spas”, Taylor Technologies, Inc., 2014, page 2 (of 4); “Intermediate Water Chemistry & Testing Notes”, Taylor Technologies, Inc., 2013, page 4 (of 4); “Advanced Water Testing & Chemistry”, Taylor Technologies, Inc., 2015, page 3 (of 4); and “Hot Water Chemistry & Testing Notes”, Taylor Technologies, Inc., 2013, pages 3 & 4 (of 4).
- Wojtowicz, John A., “A Revised and Updated Saturation Index Equation”, Journal of the Swimming Pool and Spa Industry, 3 (1), 28–34 (1998), http://jspsi.poolhelp.com/ARTICLES/ JSPSI_V3N1_pp28-34.pdf
- ANSI/APSP-11 American National Standard for Water Quality in Public Pools and Spas, Appendix A7.5—Langelier Saturation Index, Pool and Hot Tub Alliance, Alexandria, VA, 2009.
- PHTA Recreational Water Quality Committee, “TDS Fact Sheet”, Pool and Hot Tub Alliance, Alexandria, VA, 2015. http://www.apsp.org/ resources/fact-sheets.aspx
- The Association of Pool & Spa Professionals, Service Tech Manual, 4th Edition, pp 3-29 and 3-30, Alexandria, VA, 2010.
- Clark, R.M. and Boutin, B.K., Controlling Disinfection By-Products and Microbial Contaminants in Drinking Water, EPA/600/R-01/110, December 2001.
- Potts, D.E., Ahlert, R.C., and Wang, S.S. A critical review of fouling of reverse osmosis membranes. Desalination, 36:235-264 (1981).
- Reiss, C.R., Taylor, J.S., Owen, C., and Robert C. Diffusion-controlled organic solute mass transport in nanofiltration systems. Proceedings, American Water Works Association Water Quality Technology Conference, Tampa, FL., October 1999.
- Allgeier, S.C. and Summers, R.S. Evaluating NF for DBP control with the RBSMT. Journal of the American Water Works Association, 87(3):87-99 (1995).
- Cluff, C.B. Portable Swimming Pool Reverse Osmosis Systems. The Journal of the Swimming Pool and Spa Industry, 1(1):21-24 (1995).
- Kluepfel, A.M., Glauner, T., Zwiener, C., Frimmel, F.H. Nanofiltration for enhanced removal of disinfection by-product (DBP) precursors in swimming pool water retention and water quality estimation. Water Science and Technology, 63(8):1716-1725 (2011).
- Symons, J.M., Stevens, A.A., Clark, R.M., Geldreich, E.E., love, Jr., O.T., and DeMarco, J. treatment Techniques for Controlling Trihalomethanes in Drinking Water. EPA-600/2- 81-156, Environmental Protection Agency, Cincinnati, OH (1981).
- Cavestri, Richard C. and Seeger-Clevenger, Donna, “Chemical off-gassing from indoor swimming pools”, ASHRAE Transactions, RP1083, LO-09-47, 2009, 115 (2):502-512. http:// www.thefreelibrary.com/_/print/PrintArticle. aspx?id=217848230
- U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Energy Saver, https://www.energy.gov/energysaver/ swimming-pool-covers
The PHTA Recreational Water Quality Committee
Jody O’Grady, Taylor Technologies, Inc., Chair
Terry Arko, HASA, Inc.
Kevin Cox, NSF International
Dr. James Egan, LaMotte Company
Philip Escobedo, Fluidra
Rich Gallo, Pure Swim, Inc.
Kenneth Gregory, Pentair Water Quality Systems
Dr. Christopher M. Kareis, Axiall, A Westlake Company
Dr. Joseph Laurino, Periodic Products, PHTA Board of Directors Liaison
Touraj Rowhani, Sigura Terry Snow, TLS Pool Service / Independent Pool and Spa Service Association (IPSSA) Representative
Dr. Roy Vore, Vore & Associates LLC
John Weber, BioLab, Inc.
Voting Alternates:
Jeff Gaulding, BioLab, Inc.
Ellen Meyer, Sigura
Consultants: Jana Auringer, Pebble Technology International
Beth Hamil, MicroPlasma Ozone Technologies, Inc.
David Oxley, Pinellas Aquatic Consultation and Education
Dr. Stanley Pickens, Swim-Chem Consulting Services, LLC
This article first appeared in the February 2022 issue of AQUA Magazine — the top resource for retailers, builders and service pros in the pool and spa industry. Subscriptions to the print magazine are free to all industry professionals. Click here to subscribe.