Back in the late 1990s, I ran a retail pool supply store in Levittown on Long Island. One day, I picked up the mail (this was before the internet became a thing), and one of the letter had a return address of BBB. Concerned, I opened it even before I got to my desk. To date, this is the only complaint I have ever had filed against me with the Better Business Bureau. It read somewhere along the lines of this:
I just opened my pool for the season and brought a water test over to Rudy at Swimsation Pools & Billiards (name changed to protect the guilty). Rudy tested my water and did the analysis. One of the things he told me I needed was 30 pounds of alkalinity increaser. I had never had to use this alkalinity increaser before, so I became leery and didn’t buy anything from him.
I went home and added 20 pounds of baking soda like I do every year and brought a sample to a different pool store the next day. Rudy either does not know what he is doing, or he is perpetuating a scam because it turned out that I only needed 10 pounds of alkalinity increaser.
(Of course, we know that my customer had added 20 pounds of a common household substance that works as an alkalinity increaser, thereby reducing the amount of commercial alkalinity increaser he would need. Proving once again that a little knowledge can actually be a harmful thing.)
Green, blue, clear, turbid — whatever the appearance — nearly every pool we open this month and next will have one thing in common. The total alkalinity (TA), or carbonate alkalinity, will be significantly lower than when we closed the pool.
The anticipated chemistry changes over this hibernation period can be attributed to precipitation and snowmelt, which dilute the dissolved solids in the pool water. The pH of rainwater is low — typically 5.0 to 5.5 — and will drive down the pH of the water in the pool. (It’s not “acid rain,” which you hear referred to at times, usually in more industrialized areas. That has a much lower pH — about 4.0.)
TA serves as a buffer against that tendency, and helps to prevent or slow a downward drift in pH. Each buffer type has a unique pKa value that can best be considered the perfect pH for that buffer (in my customer’s case, a bicarbonate buffer).
The pKa is the pH where we have just as much of our weak acid in our buffer system as our conjugate base. This is important because the ability to buffer depends on that buffer system’s ability to either donate or accept a proton. That said, the components of the buffering system, in this case, bicarbonate ions (HCO3–), neutralize that acidic something but use a bit of itself up in the process. This is why a dose of HCl will lower both the pH and the total alkalinity, but the higher the TA, the larger the quantity required to affect the pH.
The pKa value for the bicarbonate buffer in the previous example is 6.1, which means it will have its strongest ability to buffer (one up, one down) from a pH of 5.1 to 7.1. Meaning, as something acidic tries to lower the pH towards 7.1 and below, the buffering effect becomes stronger, and the more of that acidic something it takes.
With an acceptable pH range of 7.2 to 7.8, a bicarbonate buffer system works perfectly for our needs. But I need you to remember that even though TA works best at preventing a drop in pH, it will also work to avoid an increase. This will be important coming up.
You already know the order of application where we correct the total alkalinity before correcting the pH or anything else, and I do not want to deviate from this practice. Typically, when both the TA and the pH are low, we use soda ash (sodium carbonate) at a dose of 16 ounces per 10,000 gallons to increase the total alkalinity by 11.278 ppm. That same dose would result in a 0.5 increase in pH.
Once the pH reaches the desired level, if the TA is still low, or if the total alkalinity was the only low property in the first place, we would use baking soda (sodium bicarbonate) at a dose of 16 ounces per 10,000 gallons to recognize a 7.14 ppm increase in TA. That 16-ounce dose of baking soda in 10,000 gallons of pool water would not likely cause the pH to increase by an easily measurable amount.
What if, instead of following the norm, we did something incredibly forward thinking and put our pools into a better position for opening next year and a better place for the entire pool season? You’ve heard of the borate plaster start-up (in the mix of calcium start-ups, bicarbonate start-ups, NPC start-ups, and hot starts); I want to introduce the Borate Opening.
Instead of using sodium carbonate or sodium bicarbonate to bring the total alkalinity to the desired level, we will use sodium tetraborate pentahydrate at a rate of 2.5 pounds per 10,000 gallons of pool water for a 10 ppm increase in total alkalinity (not to exceed 28.5 pounds per 10,000 gallons of pool water). Or, Borax (sodium tetraborate decahydrate) at a rate of 3.3 pounds per 10,000 gallons for a 10 ppm increase in TA, not to exceed a total of 37 pounds per 10,000 gallons. Of course, this dose assumes the borate level was at 0 ppm. You’ll likely need to add an amount of acid afterward to bring the pH back into check. Still, this addition of HCl will ‘activate’ the sodium tetraborate pentahydrate, giving us the beginnings of a Borax/HCl buffer system.
As always, I need to be able to test for anything I put into the pool. This means I need a borate test, and you’ll most likely be able to find strips easily. The dose of tetraborate we added to increase the TA to 90 ppm was not likely enough to hit our sweet spot of 50 ppm for borate. We can shift gears here slightly and switch to boric acid, which will NOT increase the pH or TA, to tweak our level at a dose of 4.77 pounds per 10,000 gallons for a 10 ppm increase in borates. This doesn’t need to be done at the opening time, but to maximize benefits, should be completed by Memorial Day.
With all of the wackadoo availability and pricing of chlorine products and muriatic acid we have seen over the past few years, the advantage of a borate level is a no-brainer.
- Aids in preventing algae growth
- Stretches chlorine supply by preventing (not killing) algae growth
- Aids in slightly slowing the rate of UV degradation of HOCl (hypochlorous acid) (notably less so than with cyanuric acid)
- Makes the water feel ‘silkier’
- Gives the water that crisp, clear, Caribbean ‘sparkle’
- Helps to reduce scaling (by binding with cations like calcium)
On top of all that, we mainly use borates as a buffer in a swimming pool to prevent the pH from going up. (Remember, a buffer slows pH movement in either direction, up or down). So, not only are we preparing for a season of uncertain chlorine and acid supply, we are also preparing our pools for both winterizing and the following spring opening.
When we get to closing and begin to balance the water, I don’t prescribe the ‘jack up the calcium hardness’ practice. I’m not a fan of putting myself in a position where draining the pool is necessary to counter an adjustment I have made to the LSI (Langeleir Saturation Index) come spring.
Besides, as discussed in my last AQUA article, a swimming pool with a calcium hardness level of more than 277.25 ppm will provide an environment more conducive to mustard algae (diatom) growth. Instead, I’ll balance my water to the desired LSI with freezing temperatures in mind by riding my pH and TA on the higher side. Just as bicarb as a buffer prevents a downward drift in pH, a borate buffer prevents the pH from climbing.
The perfect pH, or the pKa value of borates, is 9.1. This, too, is a one- up, one-down scenario with strong buffering ability at a pH of 8.1 to 10.1 which means as the pH approaches 8.1, it will become more challenging for the pH to increase. Again, this nicely bookends the top end with the pH range we want to maintain in swimming pools.
A borate buffer system works slightly different than a bicarbonate buffer, which is used up at lower pH, where the carbonic acid forms dissolved CO2, which is lost by evaporating out of the water, thereby losing part of the buffer. Borate does not diminish as it buffers. Instead, accepting a hydroxide ion changes itself into something else. Boric acid (H3BO3) takes a hydroxyl ion (OH–) from the water to form its conjugate base, tetrahydroxyborate (BH4O−4). As long as we have tetrahydroxyborate in the water, and we will as long as the pH remains above 7.0, we can deaden the impact of the (acidic) rain or snowmelt on the pH. (Refer to the inset calculation below.)
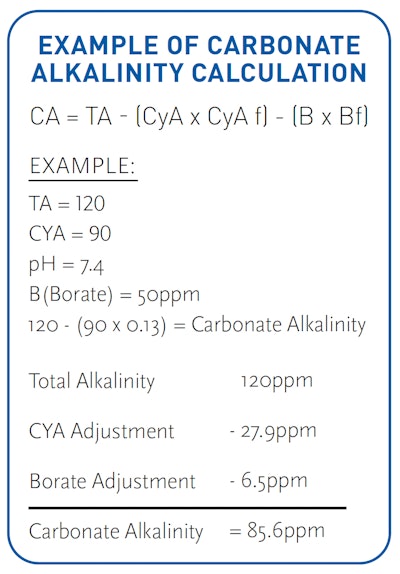
The borate level we have established, combined with a pH and total alkalinity on the higher side of the acceptable range but still balanced to the desired LSI when closing, will put us in a better position to maintain that TA throughout the winter months. Because borates aid total alkalinity in preventing a downward drift, less TA, more specifically carbonate alkalinity (the measure of bicarbonates, carbonates and hydroxides), will use itself up in preventing a decline in pH. (Carbonate alkalinity is calculated by subtracting the cyanuric acid adjustment from the total alkalinity (TA – (CYA x CYA factor) = CA (carbonate alkalinity). Carbonate alkalinity trumps total alkalinity and is the value we should use for calculating the desired LSI. Note: There is also a borate correction factor dependent upon pH, just like the CYA adjustment factor, for calculating the CA. Though, the borate correction factor is typically smaller than the CYA correction. See table below.
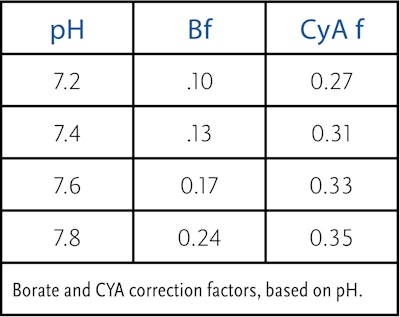
Now, consider that cyanuric acid also buffers against a downward drifting pH, though nowhere near as well as bicarbonate. Ensuring we have a minimum of 30 ppm here, in addition to the above, will increase the odds of having a carbonate alkalinity within the desired range upon opening. Increasing the CH (calcium hardness) level accounts for the temperature drop but does not consider the inevitable decline in carbonate alkalinity and pH.
The beauty of bicarb is that it is inexpensive, but borate lasts forever. Borate is similar to calcium hardness, cyanuric acid, or total dissolved solids. The only means of lowering the level is by replacing water, either wholly or partially, either intentionally or by splash out, drag out, a leak or backwashing. Before adding borates to pool water, add a chelating or sequestering agent if metals are present. Adding a large dose all at once or any amount in clumps may result in staining (by raising the pH and precipitating metal ions). Ensure the total alkalinity is less than 140 ppm, the pH is less than 7.4, and the calcium hardness level is below 350 ppm before addition. DO NOT exceed a 50 ppm borate level in swimming pools.
Now we have a swimming pool where we are preventing algae growth during both the closed and open seasons, and using less chlorine because of that. Aesthetically, the pool looks better, and we have fewer issues with scaling. On top of that, we have set up our pools so that they are more likely to open with the pH and carbonate alkalinity in the desired range. Not just for next spring but for every spring opening to follow. How cool is that?
This article first appeared in the April 2023 issue of AQUA Magazine — the top resource for retailers, builders and service pros in the pool and spa industry. Subscriptions to the print magazine are free to all industry professionals. Click here to subscribe.